15 minutes only COVID-19 TESTING KIT
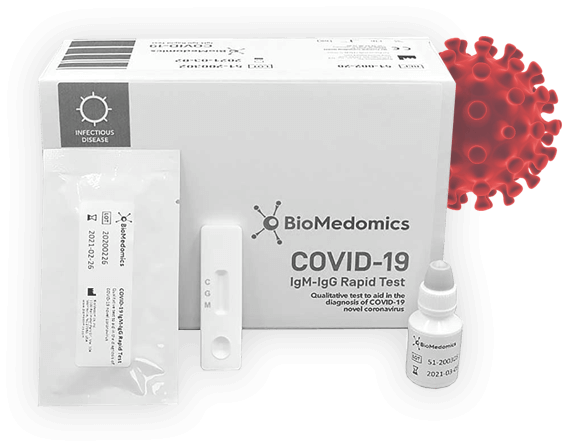
- Identifies SARS-nCoV-2 long before symptoms appear
- Test your workforce and prevent the virus from spreading
- Fast and simple finger-prick blood sample test
- One kit for one screening
- CE certified for professional and business use
- Results in under 15 minutes and four simple steps

Safeguard your employees
COVID-19 shows symptoms only 11 to 14 days after contamination. Daily screening of antibodies will help to prevent a coronavirus outbreak in your workforce at its early stages. The BioMedomics test is developed to be easily run by a company nurse or any other health personnel you have in your company.

sample

blood into test’s sample well

buffer

for the result
Results
There are four possible results in this test. The Control line ( C ) should be displayed in all of them to signify correct testing with valid results.
The Control line alone ( C ) signifies the negative result, in which there aren’t any antibodies detected in the blood sample. If C is not displayed, the test is incorrect and should be re-run.
If C and M lines are displayed, the test is valid and displays the detection of lgM antibodies. These antibodies appear in the earliest stages of COVID-19 disease.
If the G line appears alongside the quality control line C, the test is valid and positive for lgG antibodies. The lgG antibodies are present in the blood in the later stages of the Coronavirus disease.
If all lines are visible, the test is positive for lgM as well as lgG antibodies. The presence of both antibodies clearly indicates the current stage of the infection.
Professional and business use of onsite coronavirus screening
Market-leading SARS-nCoV-2 antibody detection test with results in 15 minutes
The BioMedomics IgM-IgG Combined Antibody Rapid Test is one of the first few certified tests for precise screening of the novice coronavirus infection. This accurate test is developed by BioMedomics using the successful mechanisms of the tests used by the Chinese Center for Disease Control and is available for wholesale purchases. The test is providing precise results by testing a finger-prick blood test for the presence of both LGM and lgG antibodies in the sample.

How The COVID-19 test works
The BioMedomics test for Coronavirus detects antibodies, the first flags of an infection that can be detected way before any visible symptoms. Immunoglobulin, or lgM, is the largest and most common antibody and is the organism’s first reaction to a contagion. The lgM antibody provides a wide first defense against the virus in the first days of the disease. In the next step, lgG antibodies are more precise and constructed inside the organism to fight the virus and build the long term immunity and immune memory for the virus. Testing for both lgM and lgG antibodies provides a certain identification of COVID-19 disease days before any comprehensible or visible symptoms, together with its stage, and helps to prevent further spreading within your colleagues.
- lgM antibodies indicate the earliest stages of COVID-19 contagion;
- lgG antibodies correspond to a later stage of coronavirus disease.
Thus, this combined antibody test could also provide information on the stage of infection.
Buy wholesale testsKeep your employees safe
-
What is the coronavirus test?
The COVID-19 Coronavirus screening test allows detection of the novelty SARS-nCoV-2 disease days before any outside symptoms.
-
How does the Coronavirus test work?
The BioMedomics finger-prick blood screening test identifies the lgM and lgG antibodies that correspond to different early stages of the COVID-19 disease.
-
How quick is the test?
The testing takes 15 minutes for the finger-prick blood sampling to full interpretation of the result.
-
How accurate is the test?
The BioMedomics Coronavirus test fully conforms with the Essential Requirements Annex I of the Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. The accuracy of lgM detection is 99.2%, and the accuracy of lgG detection is 99.5%.
-
How to interpret the results?
The test can display three lines. Control line C should be displayed with any successful test, and if it is alone, the test is negative. The M line corresponds to the presence of lgM antibodies, and the G line indicates the lgG antibodies in the given blood sample.
Order your coronavirus testing kit today
Buy one of the word's first rapid coronavirus tested Wholesale purchase
Wholesale purchase
